Abstract
Background: Compared with other CAR T-cell products, axicabtagene ciloleucel (axi-cel) had a shorter median wait time from leukapheresis to infusion, referred to as vein-to-vein time (real-world: axi-cel, 28 d vs tisagenlecleucel, 45 d; clinical trial: lisocabtagene maraleucel, 37 d; Riedell et al. Transplant Cell Ther 2022; Abramson et al. Lancet 2020). A study based on the JULIET trial suggested that reduced wait time is associated with increased efficacy (Chen et al.Value Health 2022). Here, we assessed the real-world impact of vein-to-vein time on outcomes of axi-cel in r/r LBCL.
Methods: A total of 1383 patients from 78 US centers treated with commercial axi-cel for r/r LBCL between October 2017 and August 2020 were identified from a non-interventional post-authorization safety study using the Center for International Blood and Marrow Transplant Research (CIBMTR) registry. Patients with the following were excluded: primary central nervous system lymphoma or lymphoma other than LBCL, prior non-transplant cellular therapy, missing data on comorbidity (Sorror et al. Blood 2005), unknown or outlying date of leukapheresis (≤2 d before lymphodepleting [LD] chemotherapy or ≥144 d before infusion), or no follow-up. Effectiveness outcomes were overall and complete response rates (ORR and CR rates), duration of response (DOR), and progression-free and overall survival (PFS and OS). Adverse events of interest included cytokine release syndrome (CRS, Lee 2014 criteria), immune effector cell-associated neurotoxicity syndrome (ICANS, ASTCT criteria), and prolonged neutropenia and thrombocytopenia. Odds ratios (ORs) and hazard ratios (HRs) were estimated using logistic and Cox regressions after adjustment of key prognostic factors such as age, comorbidities, ECOG performance status, disease characteristics at diagnosis, and bridging therapy (Table 1). Adjusted curves were generated based on the direct adjusted survival function (Makuch J Chronic Dis 1982).
Results: Overall, median vein-to-vein time (from leukapheresis to infusion) for axi-cel was 27 d (interquartile range [IQR], 26-32 d), including a median of 5 d (IQR, 5-5 d) from start of LD chemotherapy to infusion. Vein-to-vein times were consistent regardless of the following baseline characteristics: disease histology, sex, race, ethnicity, ECOG performance status before infusion, or chemo-sensitivity. Patients with shorter vein-to-vein times appeared to be younger and less likely to have comorbidities (Table 1). Patients with vein-to-vein time of ≥40 d were more heavily pretreated and more likely to receive bridging therapy.
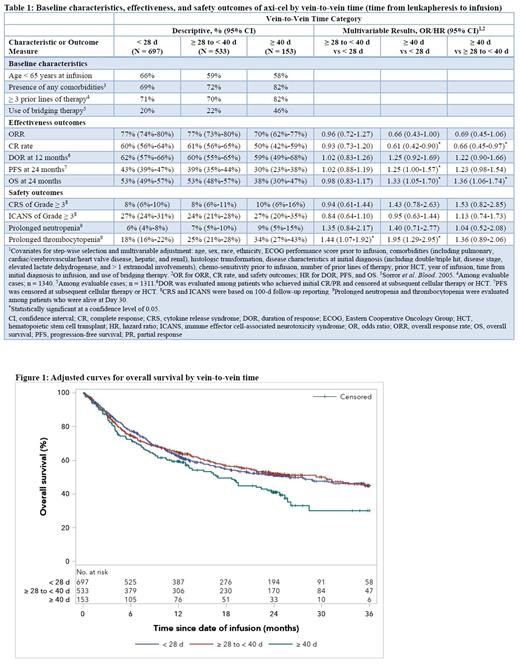
At a median follow-up of 24.2 months, better outcomes were observed in patients with shorter vein-to-vein times. CR rates were 60%, 61%, and 50% (ORR 77%, 77%, and 70%) for patients with vein-to-vein time of <28 d, ≥28 to <40 d, and ≥40 d, respectively. OS at 24 months was 53% for patients with vein-to-vein time of both <28 d and ≥28 to <40 d, vs 38% for those with ≥40 d wait time. After other key prognostic factors were adjusted, patients with vein-to-vein time of ≥ 40 d had significantly lower CR rate and OS compared with patients with <28 d (OR 0.61 [95% CI 0.42-0.90] for CR; HR 1.33 [95% CI 1.05-1.70] for OS) and ≥28 to <40 d wait time (OR 0.66 [95% CI 0.45-0.97] for CR; HR 1.36 [95% CI 1.06-1.74] for OS).
CRS of any grade or Grade ≥3 and prolonged neutropenia were consistent regardless of vein-to-vein time. Patients with vein-to-vein time of <28 d had more ICANS of any grade compared with those with ≥28 to <40 d wait time (OR 1.34 [95% CI 1.06-1.71]), while ICANS of Grade ≥3 was not significantly different between the two groups (Table 1). Among patients alive at Day 30, those with vein-to-vein time of ≥28 to <40 d and ≥40 d had higher rates of prolonged thrombocytopenia compared with those with <28 d wait time (OR 1.44 [95% CI 1.07-1.92] and 1.95 [95% CI 1.29-2.95], respectively).
Conclusions: In this real-world analysis, most patients with r/r LBCL received axi-cel infusion within 5 weeks after apheresis. Shorter vein-to-vein time was associated with a favorable CR rate, OS, and reduced risk of prolonged thrombocytopenia even after adjustment of key prognostic factors; however, ICANS of any grade may be higher. Overall, these findings highlight the importance of shortening vein-to-vein time in patients treated with axi-cel.
FLL and Z-HH contributed equally.
Disclosures
Locke:BMS: Research Funding; Takeda: Consultancy; Sana: Consultancy; Daiichi Sankyo: Consultancy; Clinical Care Options Oncology: Other: Education or editorial activity; Imedex: Other: Education or editorial activity; Society for Immunotherapy of Cancer: Other: Education or editorial activity; CAREducation: Other: Education or editorial activity; Aptitude Health: Other: Education or editorial activity; BioPharm Communications: Other: Education or editorial activity; ASH: Other: Education or editorial activity; CERo Therapeutics: Research Funding; ), National Cancer Institute: Research Funding; Leukemia and Lymphoma Society: Research Funding; A2: Consultancy; Celgene: Consultancy; Other: Patents & Royalties: patents, royalties, other intellectual property from several patents held by the institution in my name (unlicensed) in the field of cellular immunotherapy.; Wugen: Consultancy; Umoja: Consultancy; Novartis: Consultancy, Research Funding; Legend Biotech: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Janssen: Consultancy; Iovance: Consultancy; GammaDelta Therapeutics: Consultancy; Emerging Therapy Solutions Gerson Lehrman Group: Consultancy; EcoR1: Consultancy; Cowen: Consultancy; Calibr: Consultancy; Cellular Biomedicine Group: Consultancy; Bristol Myers Squibb/Celgene: Consultancy; Bluebird Bio: Consultancy, Research Funding; Allogene: Consultancy, Research Funding; Amgen: Consultancy. Hu:Kite, a Gilead Company: Current Employment. Siddiqi:TG Therapeutics: Research Funding; Oncternal: Research Funding; Seattle Genetics: Speakers Bureau; Janssen: Speakers Bureau; PCYC: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Kite, a Gilead Company: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Juno: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Jacobson:Humanigen: Consultancy, Honoraria, Other: Travel Support; Ispen: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; bluebird bio: Consultancy, Honoraria; Instil Bio: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Pfizer: Other: Travel Support, Research Funding; Lonza: Consultancy, Honoraria, Other: Travel Support; Clinical Care Options: Speakers Bureau; ImmPACT Bio: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Celgene: Other: Travel Support; Axis: Speakers Bureau; Nkarta: Consultancy, Honoraria; Precision BioSciences: Consultancy, Honoraria, Other: Travel Support; Novartis: Consultancy, Honoraria, Other: Travel Support; BMS/Celgene: Consultancy, Honoraria; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding. Nikiforow:Kite, a Gilead Compnay: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees. Ahmed:Chimagen: Consultancy, Research Funding; Seagen: Research Funding; Merck: Research Funding; Servier: Membership on an entity's Board of Directors or advisory committees; Xencor: Research Funding; Myeloid Therapeutics: Consultancy; Tessa Therapeutics: Consultancy, Research Funding. Miklos:Adaptive Biotech: Consultancy; Pharmacyclics: Patents & Royalties: cGVHD Ibrutinib patent ; Allogene: Research Funding; Bristol Meyers Squibb: Consultancy; Fosun Kite: Consultancy, Honoraria; Novartis: Consultancy; Kite, a Gilead Company: Research Funding; Janssen: Consultancy, Honoraria. Lin:Bluebird Bio: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Sorrento: Consultancy; Legend: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Juno: Consultancy; Novartis: Consultancy; Vineti: Consultancy; Merck: Research Funding; Takeda: Research Funding; Gamida Cell: Consultancy. Lunning:EUSA: Consultancy; Kite, a Gilead Company: Consultancy; Morphosys: Consultancy; Genmab: Consultancy; Genentech: Consultancy; BMS: Consultancy, Research Funding; Fate Therapeutics: Consultancy; Astellas: Consultancy; TG Therapeutics: Consultancy; ADC Therapeutics: Consultancy; Diiachi-Sankyo: Consultancy; Acrotech: Consultancy; AbbVie: Consultancy; Janssen: Consultancy; Nurix Therapeutics: Consultancy; Astra-Zeneca: Consultancy; Pharmacyclics: Consultancy; Seattle Genetics: Consultancy; CURIS: Research Funding. Hill:BMS: Consultancy, Honoraria, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Ghobadi:Amgen: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Atara: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy; Wugen Inc: Consultancy. Miao:Gilead Sciences: Current holder of stock options in a privately-held company; Kite, a Gilead Company: Current Employment. Shahani:Amgen: Speakers Bureau; Kite, a Gilead Company: Current Employment, Current holder of stock options in a privately-held company; City of Hope: Other: NA; American College of Obstetrics and Gynecology: Consultancy. Spooner:Delta Hat Limited: Other: Support with conception, analysis and manuscript writing. Commissioned by Kite, a Gilead company; Gilead Sciences: Current holder of stock options in a privately-held company; Kite, a Gilead Company: Current Employment. Fu:Kite, A Gilead Company: Current Employment, Current holder of stock options in a privately-held company; Amgen: Current holder of stock options in a privately-held company; Cellares: Patents & Royalties: Intellectual property. Patel:Kite, A Gilead Company: Current Employment, Current holder of stock options in a privately-held company. Xu:Kite, a Gilead Company: Current Employment. Pasquini:Bristol Myers Squibb: Consultancy, Research Funding; Novartis: Research Funding; Kite: Research Funding; Janssen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.